- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
-
Services
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Physician & Clinical Bio-Skills Training
- Cadaver Testing & Training
- Histopathology & Analytical Testing
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
Services
Your Human Cadaver Studies Facility for Medical Device Testing and Physician Training
Do you need to schedule a bio-skills training or R&D study in a human cadaver model?
Surpass can help. Our team has proven experience working with human cadaver models for 1) physician and clinical bio-skills training and 2) medical device product development testing. Surpass' skilled staff operates as an extension of your team, precisely preparing the cadaver specimen exactly as you need. Our team can help with:
- Cannulating vessels
- Performing specialized specimen prep (e.g. enemas, suturing, and more)
- Assisting with prescreen imaging for size or orientation of specific anatomical structures with fluoroscopy, ultrasound, OCT, CT, and more
- Exposing or isolate desired anatomy
- Developing customized positioning devices
- Creating physiologically pressurized models
- Setting up vascular flow loops
- Excising deployed implants or tissues
- And more
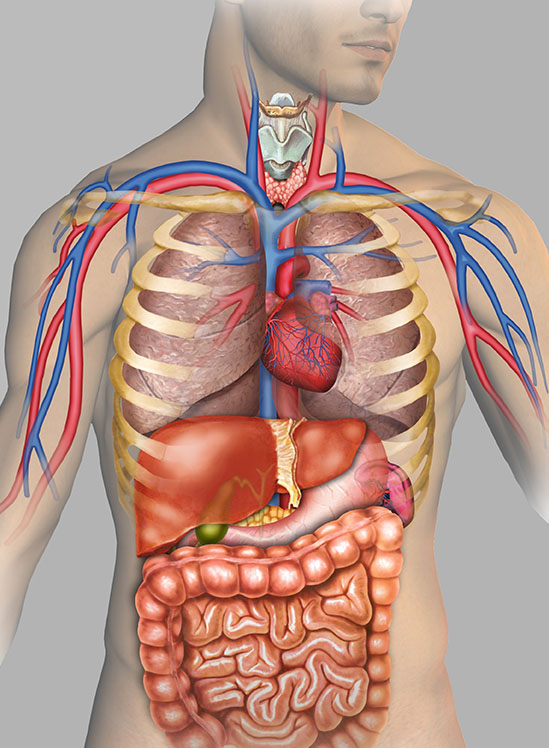
We source only serologically tested specimens from credentialed vendors and can procure human cadavers with previous placed medical devices (stents, heart valves, vascular grafts, pacemakers) and disease states of interest for your study:
- Coronary artery disease (CAD)
- Heart valve stenosis
- Obesity
- Osteoporosis
- Peripheral artery disease (PAD)
- And more
At the completion of your cadaver study, we handle the facility clean up and biological disposal and cremation according to applicable regulations. We are here to help you every step of the way - making sure your cadaver studies run smoothly from beginning to end.
When you are looking for a trusted cadaver study facility, Contact Surpass. Our team is here to support your medical device human cadaver testing and training needs.
Experienced CRO for R&D Testing and
Bio-skills Training in Cadaver Models.