- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
- Services
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
About
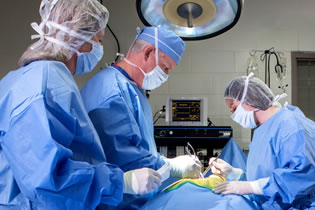
At Surpass, We Bring More to Our Client Partners
Our innovative approach to preclinical research goes beyond the ordinary. We're changing the rules for a better way to get from concept to reality.
With increasing pressures on the health care sector, every stakeholder in the medical innovation continuum needs to work in concert. Long gone are the days where engineering, preclinical research, clinical development, regulatory, and other entities operate separately. To stay competitive, the life science industry as a whole needs to be connected in ways that enable us to develop new medical solutions faster, better, and cheaper. This only happens in a true team environment where the task is supreme.

At Surpass, we bring more to our client partners. We offer insightful solutions and support every step of the way as your collaborator. Our people are a valuable extension of your product development team. We customize our preclinical research services at our greater Twin Cities location, near Minneapolis/St. Paul, to meet your specific project need.
Surpass offers a number of critical advantages that make your preclinical feasibility, product development, GLP safety and training studies successful.
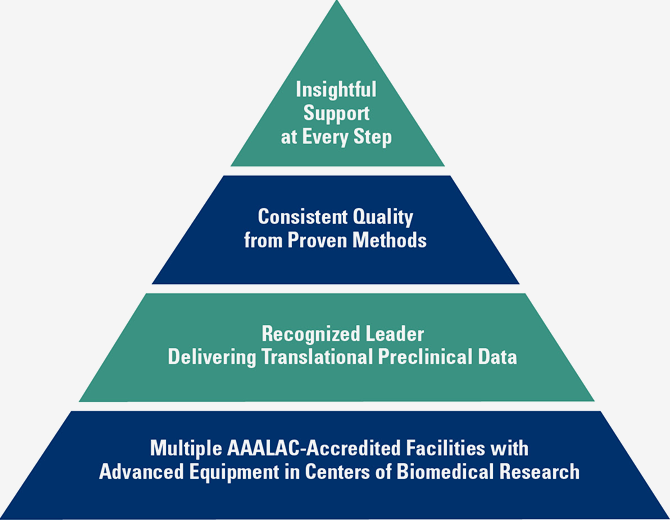
For your medical device, pharmaceutical, biologic or combination product testing, partner with a preclinical research team that surpasses all the rest. Choose Surpass.
Surpass [ser-pas] verb:
to go beyond in excellence or achievement;
be superior to; excel