- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
- Services
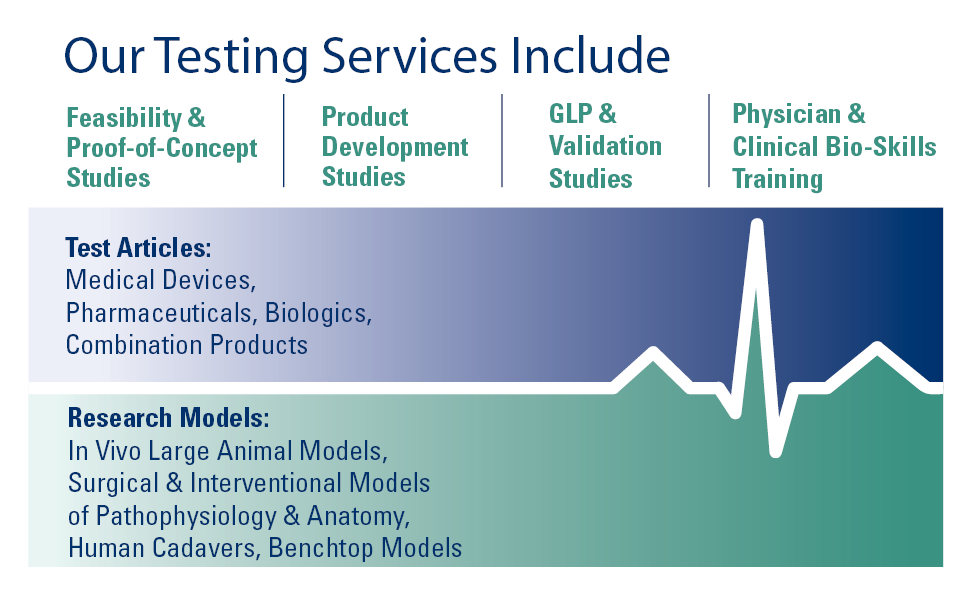
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
About
Innovative Preclinical CRO Services with Insightful Support That Advance Your Concept to Reality
Surpass is a trusted partner and contract research organization (CRO) specializing in preclinical services for medical devices, drugs, pharmaceuticals, biologics, and combination products.
Built on the foundation of a prominent preclinical CRO in the Minneapolis/St. Paul Area (greater Twin Cities), the team at Surpass has successfully serviced the global Biomedical Community for more than 20 years. Whether it is our
- Renowned reputation for regulatory excellence,
- Vital scientific know-how for efficiently conducting studies,
- Impeccable attention to animal care, or
- Solid data package for regulatory and IRB submissions,
Surpass is a globally recognized leader for preclinical research.
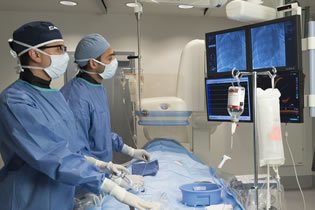
Our team has extensive experience conducting translational research in large animal models and human cadavers with specialized expertise in surgical and interventional procedures.
Surpass provides critical preclinical research data and innovative solutions through proven methods and compliance, experienced professionals and state-of-the-art facilities with advanced equipment. We are a preclinical CRO you can trust - an integral member of your product innovation team.
Whether you are a start-up or an established company, Surpass is uniquely qualified to make your preclinical study successful. Located near the center of Medical Device innovation in the greater Twin Cities/Minneapolis area, we offer insight, expert advice and the level of comprehensive support you need to make your study successful - the first time.
Make Surpass your trusted preclinical CRO partner. Contact us today.
Your Partner for
Advancing Medical Innovation
to Market.