- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
- Services
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
About
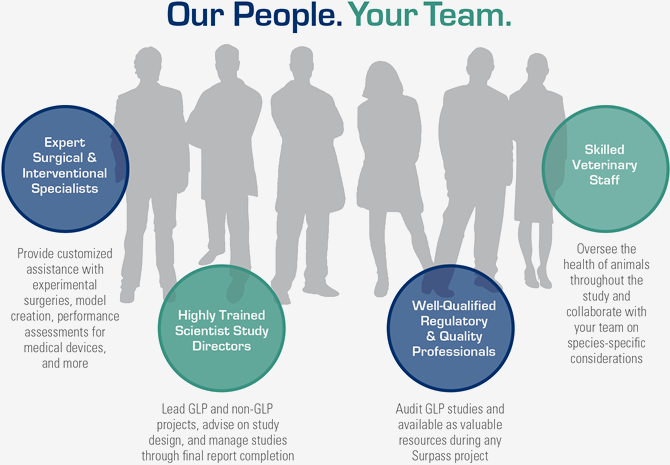
Insightful Support at Every Step
Surpass' award-winning team of scientists, veterinarians, research technicians, regulatory/quality professionals, and support staff is led by accomplished life-sciences executives and scientists, Tim Pelura, PhD, CEO and Mark Cunningham, PhD, CSO.
With hundreds of years of collective experience running successful preclinical studies for medical devices, pharmaceuticals, biologics and combination products, we can lead you through the entire process. Regardless of where you are in the product innovation cycle, our people partner with you to generate translational preclinical data and innovative solutions that advance your product to market.
We can support you with:
- Study design and model selection
- Protocol and method development
- Research model creation
- Medical device performance evaluation
- Experimental surgery
- Human factors assessment
- Report writing
- FDA / regulatory agency communication
Whether you need us to perform the experiment for you or support your preclinical efforts our team is here to help. Learn more about our Expertise and Testing Services.
Key Management and Personnel
Corporate Management
President and CEO, PhD
Chief Scientific Officer, PhD
Test Facility Management and Sr. Director of Operations, MS, RQAP-GLP
Manager of Regulatory Compliance (and Quality Assurance), AA, CVT, RQAP-GLP
Key Scientific & Veterinary Team Members
Associate Scientific Director, BS
Scientist/Study Director, BS
Director of Surgical and Interventional Services, BS, SRS
Surgeon and Interventionalist, AS, LAT, SRS
Attending Veterinarian, BSChE, DVM
Surpass.
Your Preclinical Team.






