- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
- Services
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
Preclinical Blog
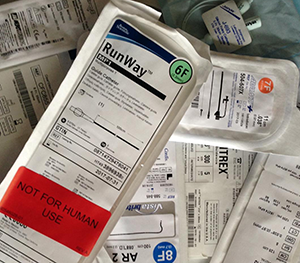
Test Article Labels for a Medical Device or Combination Product GLP Preclinical Study
Oct 30, 2014
How should test and control articles be labeled for a medical device or combination product GLP preclinical study?
Making sure test and control articles are labeled properly is of key importance for the success of a GLP study. Appropriate labeling prevents mix ups, supports that the article is as described in the protocol, and communicates to the contract research organization (CRO) how articles should be safely stored to prevent deterioration prior to testing.
Label each storage container (package) with the following information at a minimum:
- Name of the article
- Identifying number(s), e.g., lot number, batch number, reference number
- Expiration date
- If the article contains more than one component and those components have separate expiration dates, the earlier expiration date should be listed on the article
- If an expiration date has not been established, labeling options include:
- An expiration date that reflects preliminary results or accelerated testing
- Indication that the expiration date has not been established / determination of the expiration date is in process
- Storage Conditions
When planning an FDA Good Laboratory Practice (GLP) preclinical study for a medical device or combination product, it is important to consider the strategy for dealing with test and control article requirements early in the process, to ensure appropriate documentation of article build, packaging, sterilization, and testing, as well as retention of appropriate reserve samples. As your trusted preclinical partner, Surpass is here to help you through the entire GLP process. We will be posting additional entries in our blog with hints for planning a GLP preclinical Study.
Next Steps
- Sign up to receive notification of future blog posts by Surpass.
- View other blog posts related to Planning a GLP Study.
- Learn more about Surpass' GLP preclinical testing services.
- Contact us to learn more about medical device, pharmaceutical, biotech, or combination product GLP studies.
- Download our current Whitepapers on Preclinical In Vivo Study Design and Choosing a Preclinical CRO.




