- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
-
Services
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
Services
A Preclinical CRO with Extensive Good Laboratory Practice (GLP) Study Experience to Steer You Through the Process
Surpass has developed an international reputation as a GLP-compliant lab and the preclinical CRO-of-choice to conduct GLP studies for supporting successful regulatory and clinical submissions. We provide a proven quality system and knowledgeable personnel to partner with your team every step of the way to ensure a successful study.
With hundreds of GLP preclinical studies under our belt, positive FDA inspection results, and technical expertise across numerous medical disciplines, Surpass can guide you through the entire process. Our support includes assistance with study planning and design, FDA protocol review meetings, protocol development, control article and medical product acquisition, study execution, reporting, and beyond, to providing help with questions from regulatory agencies about the preclinical study results.
View our presentation below with helpful hints for planning your GLP study.
Contact Surpass today to discuss your upcoming GLP preclinical study with our award winning research team. We've assisted many medical device, pharmaceutical and biotech companies translate their product into the clinical setting through successful GLP studies, we can do the same for you.
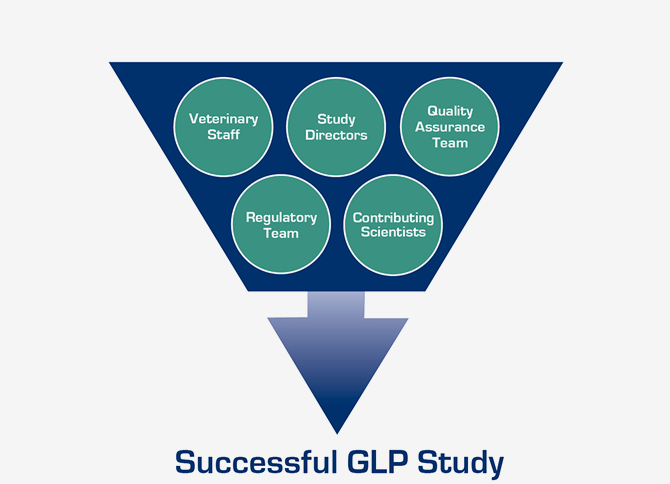
GLP Study Experience
You Can Count On.