- About
- Expertise
- Expertise Overview
- De Novo & Disruptive Technologies
- Cadaver Testing & Training
- Physician & Clinical Bio-Skills Training
- Histopathology & Analytical Testing
- Cardiac (Structural Heart & EP)
- Dermal
- Gastrointestinal
- General & Reconstructive Surgery
- Neurological
- Oncology
- Orthopedic/Spine
- Respiratory
- Urogenital
- Vascular
- Wound Healing/Traumatic Injury
-
Services
- Testing Services Overview
- Research Models
- GLP Studies & FDA Assistance
- Alprazolam (Xanax)
- Armodafinil (Nuvigil)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Ivermectin (Stromectol)
- Lorazepam (Ativan)
- Modafinil (Provigil)
- Molnupiravir (Lagevrio)
- Prednisone (Deltasone)
- Pregabalin (Lyrica)
- Sumatriptan (Imitrex)
- Testosterone (Cernos)
- Zolpidem (Ambien)
- Zopiclone (Imovane)
- Support
- News/Events
- Blog
- Contact
Services
Preclinical Testing Services That Advance Your Innovations
Is your novel therapy a medical device, pharmaceutical, biologic, or combination product? We can help with preclinical testing services throughout the innovation and product development cycle.
Surpass specializes in studies that involve human cadavers and large animal research models. Our veterinarians and scientists work as an extension of your product development team, offering customized support to meet your project needs.
We provide assistance in selecting the right animal model for your project, support in developing GLP and Non-GLP protocols and reports, and expertise in performing device evaluations. Our preclinical testing services encompass early-stage feasibility projects through GLP Preclinical Safety Studies and Physician Training.
Feasibility/Proof-of-Concept Studies
We can help you propel ideas into product development. Throughout the years, Surpass has worked with life science entrepreneurs to deliver vital preclinical study data that allows them to secure early-stage funding or facilitate a successful technology transfer. Our preclinical testing team can advise on model selection and preclinical study design, maximizing your results at a reasonable cost.
The skilled surgeons and interventionalists at Surpass provide valuable feedback on early stage prototype performance. Our scientists and veterinarians have assisted many serial entrepreneurs and first-time innovators in choosing the right methods for testing their medical devices and biologic prototypes and addressing fundamental questions. We can do the same for you.
Product Development Studies
Surpass is proud to have supported hundreds of successful product development teams over the years - performing early prototype evaluations, iterative testing, end-user validation, GLP preclinical studies, and human factors testing. We support efforts in acute and chronic in vivo, human cadaver, and bench top models.
In addition, Surpass has long been known as the "go-to" lab for GLP testing services. Our scientists and regulatory team have the skills and experience necessary to be a valuable partner on your upcoming product development project.
Surgical and Interventional Services
Surpass' skilled professionals can create surgical and interventional models of specialized anatomy or human disease states for testing your product. Our surgeons and interventionalists have experience providing valuable feedback on product performance and optimizing treatment procedure steps in preparation for human clinical cases. Surpass can provide surgical assistance for your physician advisor, or perform the experimental surgery for you. We tailor our support to meet your project needs.
Clinical Products for Studies
If you are in need of currently available products to serve as study controls, Surpass can order most clinically available products that are approved in the United States. Let us know how we can help you.
Grant-Funded Projects
Surpass has worked on government-funded research for many years, and has an OLAW Assurance and a DOD Facility Safety Plan to perform this type of work. Our scientists can support your grant writing, and will provide the appropriate Institutional Animal Care and Use Committee (IACUC) documentation necessary to successfully file with the funding agency. To learn more about how we can support your grant-funded projects, contact one of our scientists today.
Histopathology and Bioanalytical Testing
Surpass has partnered with experts in the fields of histopathology and analytical testing. Whether your specific need is plastic embedding, immunohistochemistry, or something unique, we are happy to set up introductions or coordinate these efforts for you.
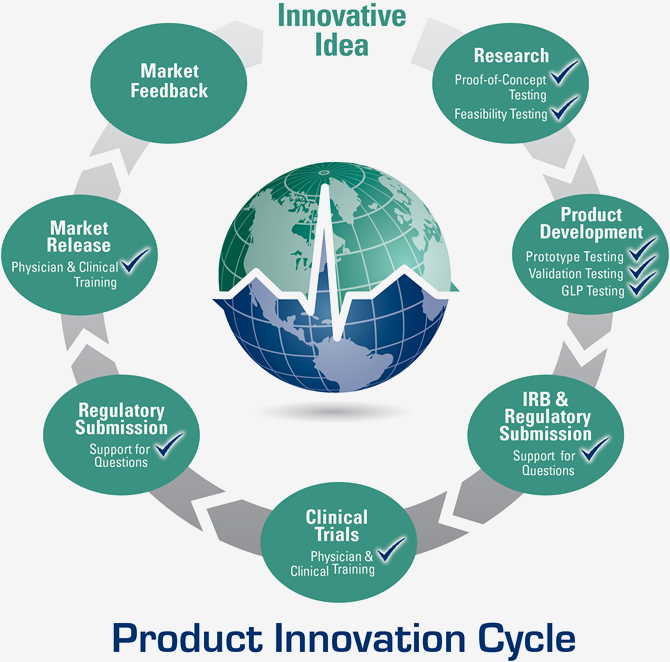
Preclinical Testing Solutions
Throughout the
Product Innovation Cycle.